IV. Botany
A. Early Pollen Research
Unbeknownst to Brown, the mechanism of fertilization of the ovule by pollen he had been looking for had been observed by accident in 1822 by the Italian optical designer, astronomer and botanist Giovanni Battista Amici (1786-1863). Amici was looking at a hair on a stigma:(1)
I happened to observe a hair with a grain of pollen attached to its tip which after some time suddenly
 |
| Giovanni Battista Amici (portrait by M. Gordigiani, Palazzo Pitti) |
exploded and sent out a type of transparent gut. Studying this new organ with attention, I realized that it was a simple tube composed of a subtle membrane, so I was quite surprised to see it filled with small bodies, part of which came out of the grain of pollen and the others which entered after having traveled along the tube or gut.
Thus, what is now called the pollen tube was discovered.
Brown became aware of this, and launched an investigation of the germination of pollen grains in orchids in 1831. He was the first to realize that contact with the stigma causes the pollen to germinate, and he observed the particles from
within the pollen flowing into the pollen tube. (Ironically, if in his 1827 work he had decided to observe pollen in vivo, instead of in vitro, he likely would have seen the pollen tube, pursued that, and his Brownian motion observations might never have taken place.) By 1833, he had published his observations that the pollen tube reaches and penetrates the ovule. Brown also was the first to insist upon the importance of insects in pollination.(2)
During this 1831 work, Brown noticed a structure within cells belonging to an orchid leaf's epidermis (its outer layer). Characteristically he first looked at the internal cells of the plant and made the same observation, and then studied a myriad of other plants, with the same result. Brown had found the cell nucleus, which he named (from the latin word for ``little nut"). He also specified that the pollen grain contains a nucleus. [One wonders if the term arose in physics due to Brown. Michael Faraday (1791-1867) used it to describe the center of the atom in 1844: Faraday knew Brown, lectured about Brownian motion in 1829, and letters are extant from Faraday to Brown. The term was adopted by Ernest Rutherford in 1912: one wonders how that came about.]
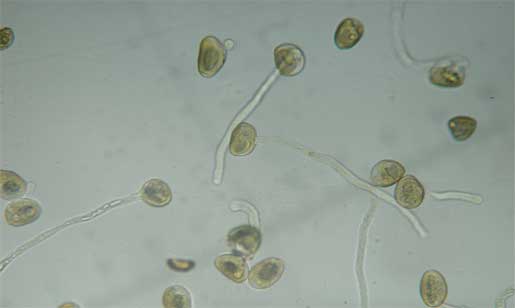 |
| Pollen tubes |
As we have seen, a pollen grain also contains amyloplasts and spherosomes (starch and fat organelles). Their nature became known within a decade after Brown's publications, but much was still unknown and wrongly conjectured. The two types of particles seen by Brown were described by John Lindley (who earlier had a falling out with Brown, following an article Lindley wrote that was construed to attribute some of Brown's early work to Bauer) in his 1848 book An Introduction to Botany:(3)
In consequence of their manifest motion it has been conjectured that the longer particles of the fovilla were the incipients of the embryo, and it is by the the introduction of one or more of these into the ovule that the act of impregnation is accomplished by the deposit of a rudimentary embryo in the ovule. [Wrong!] But both Fritzsche and Mohl agree in considering many of the smaller particles of the fovilla as minute drops of oil: [Right!] the molecular motion has been ascribed to currents in the fluid, in which the fovilla is suspended, and which, according to Fraunhofer, no precautions can possibly prevent; [Wrong!] and, what is more important, the larger particles become blue upon the application of iodine, without however losing their property of motion, as Fritzsche has shown: they are therefore starch.[Right!]
Lindley cites Fritzshe's and Mohl's work, published in 1833 and 1837, respectively.
B.Pollen Physiology
A pollen grain consists of an elaborate, three-layered cell wall surrounding a single, living cell,(4) called a tube cell, because it can grow into a pollen tube. The outer layer of the pollen wall is sculptured and ornamented. It
consists mainly of a tough, water-insoluble, fatty substance called sporopollenin.(5) Because of its thickness, ornamentation and chemical nature, the pollen grain wall is often optically opaque, and many of the internal contents of fresh pollen grains remain elusive using a light microscope. (However, as we have seen, the oblong
 |
| Miscellaneous pollen |
amyloplasts of C. pulchella can be seen through the pollen wall, which led Brown to start working with that plant.) At particular locations, the cell wall is modified to form one or more apertures or pores. (C. pulchella, C. elegans and C.amoena have three pores). When pollen lands on a flower's stigmatic surface, the pollen absorbs water through the pores. Molecules (such as amino acids, oils and sugars(6),(7)) of the stigma induce the pollen to germinate, with a pollen tube emerging through one of these pores. When, in the laboratory, pollen grains are more uniformly surrounded by an artificial incubation medium, several tubes may emerge from a single pollen grain.
In spite of opacity of a pollen grain's cell wall, ultrastructural studies with an electron microscope reveal the tube cell contents.(8) There is a centrally located nucleus surrounded by the cytoplasm, which consists of a viscous fluid and its contents, membrane-bound structures called organelles (“little organs�). These organelles include amyloplasts (which store starch), spherosomes (which store lipids), numerous very small ribosomes needed for protein synthesis and a structure called an endoplasmic reticulum, which is involved in transporting proteins.
A small lens-shaped cell, the generative cell, is also found in the cytoplasm of the tube cell. This generative cell is passively transported within the elongating pollen tube and ultimately divides to form two non-motile sperm. The
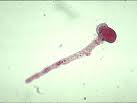 |
| Pollen tube with streaming contents |
pollen tube passes through the stigma and down the style, and reaches an ovule. The ovule is surrounded by integuments (a layer that will become the outer coating of the seed) pierced by a hole called a micropyle. The pollen tube passes through the micropyle, and enters the ovule. The ovule contains an egg cell, as well as a neighboring cell called the “central cell.� When the pollen tube arrives in the vicinity of the egg, a pore forms at the tip of the pollen tube, which bursts, and the two sperm are released.(9) A double fertilization is achieved: one sperm fertilizes the egg to form the embryo (seedling), and the other sperm nucleus unites with the nucleus of the central cell to form a unique nutritive tissue, the endosperm, which will become the “food� for the embryo or seedling.
Within a few minutes of landing on the receptive stigmatic surface of the flower (or upon being placed in an incubation medium), respiratory activity(10) (oxygen--dependent reactions that create the energy rich molecule ATP which is the energy currency of the cell) and protein synthesis(11) are initiated within the tube cell. By 15 minutes, RNA synthesis has begun and, even when this RNA synthesis is blocked experimentally, the germination and early growth of the pollen tube proceeds.(12) This suggests that the RNA required for the early phases of germination and tube growth is preformed in the pollen tube cell and is ready for utilization.
Ultrastructural studies of pollen germination and tube growth show that in the few minutes before emergence of the pollen tube, structures called Golgi bodies are activated. These accept proteins from the endoplasmic reticulum and bundle them into more complicated molecules. These molecules are shipped out (so to speak) in packages called vesicles,(13) produced by the Golgi bodies. The vesicles migrate and fuse locally with the pollen tube's boundary layer, called the plasma membrane, to form the growing tip of the pollen tube. As they fuse with the plasma membrane, the vesicles release their contents of cell wall material that contribute to the lengthening pollen tube. They also release enzymes that are believed to help dissolve a pathway for the pollen tube through the stylar tissue of the flower's pistil.(14)(15) The starch and lipid, stored in the amyloplasts and spherosomes, respectively, are presumably utilized as energy sources and provide raw materials for the construction of new cell wall material and new plasma membrane during pollen tube elongation.
When pollen grains of many plants are placed in water for microscopic examination, they often will germinate and form a short tube, but then they frequently rupture, to release the cytoplasmic contents of the tube cell into the water. As the cytoplasmic contents disperse into the water, the more numerous and larger amyloplasts and spherosomes are seen. Other organelles are too small (ribosomes are about .02 micrometer) to be seen with the light microscope or too few (nucleus) to be easily spotted.
Jost(16) first suggested that, during pollen germination and pollen tube growth, sugar plays the role of osmotically regulating the swelling and bursting of pollen grains and tubes. However, Bilderback(17) demonstrated that the pollen grains of some plants do not require sugar to stabilize pollen growth and tube elongation. Schumucker(18) recognized that boron plays an active role during pollen tube growth. Its physiological behavior remained unknown until Dickinson(19) found that boron binds in a reversible manner to growth-related sites in the pollen tube. Calcium and potassium(20)(11)(17) also have been found to be essential for stable growth of pollen tubes. Weiseseel and Jaffe(21) were able to show that potassium enters the tips of actively growing pollen tubes. The directed growth of the pollen tube to the plant egg may be due to a gradient of calcium, potassium, hydrogen and chloride within the flower's pistil, extending from the stigma to the egg(22)(23). The details of pollen tube evolution are an active subject of research(24). Observation of pollen tube growth makes an engaging student lab.(25)
Artificial pollen incubation media did not begin to be formulated until the beginning of the twentieth century. Thus, Brown put pollen into water, observed the contents of ruptured pollen grains, and discovered Brownian motion instead of (rediscovering) the pollen tube.
----------------------------------------------------------------------------------
1) At the web site of the Scuola Normale of Pisa, http://gbamici.sns.it/eng/osservazioni, select osservazionibiologiche.htm.
2) D. J. Mabberley, Jupiter Botanicus: Robert Brown of the British Museum (J. Cramer, Braunschweig 1985).
3) John Lindley, An Introduction to Botany, Fourth edition, Volume 1 (Longman, Brown Green and Longmans, London 1848), p. 361, available at the Googlebooks site.
4) J. Heslop-Harrison, Pollen: Development and Physiology (Butterworths, London 1971).
5) A. Frey-Wyssling, Die Pflanzliche Zellwand (Springer-Verlag, Berlin 1959).
6) Y. Heslop – Harrison and K. R. Shivanna, Ann. Bot .41, 1233 (1977).
7) U. Kirsten, M. Beidermann, G. Liebezeit, R. Dawson and L. Bohm , Eur. J. Cell Biol.19, 281(1979).
8) L. L. Hoefert, Am. J. Bot. 56, 363 (1969).
9) W. A. Jensen and D. B. Fisher, Planta 84,158(1969).
10) J. Tupy, Biol. Plant. 4, 69 (1962).
11)J. P. Mascarenhas and E. Bell, Biochim. Biophys. Acta 179, 199 (1969).
12) J. P. Mascarenhas, Am. J. Bot. 53, 563 (1966).
13) W. G. Rosen, S. R. Gawlik, W. W. Dashek and K. A. Siegesmond, Am. J. Bot. 51, 61 (1964).
14) W. W. Dashek and W. G. Rosen, Protoplasma 61,192 (1966).
15) R. G. Stanley and H. F. Linskens, Nature 203, 542 (1964).
16) L. Jost, Ber. Deutsch. Bot. Ges. 23, 504 (1905).
17) D. E. Bilderback, Environ. Health Perspect. 37, 95 (1981).
18) T. Schumucker, Planta 23, 264 (1935).
19) D. B. Dickinson, J. Am. Soc. Hort. Sci. 103, 263 (1978).
20) J. L. Brewbaker and B. H. Kwack, Am. J. Bot. 50, 859 (1963).
21) M. H. Weisenseel and L. F. Jaffe, Planta 133, 1 (1976).
22) K. R. Robinson and M. A. Messerli, Sci. STKE 2002, pe51 (2002). The article in
this electronic journal may be downloaded from the web site of one of the authors:http://www.bio.purdue.edu/people/faculty/robinson/Lab/pollen.htm.
23)X. Chao-Tian, Q. Yi-Lan , C. Su-Hong and T. Hui-Qiao, J. Pl. Physiol. and Molec. Biol. 31, 53 (2005).
24)N. A. Eckardt, The Plant Cell 17, 327 (2005).
25) Here are some web sites that describe how to observe pollen tubes in the laboratory:
http://www-saps.plantsci.cam.ac.uk/pollen/pollen2.htm,
http://www-saps.plantsci.cam.ac.uk/worksheets/ssheets/ssheet4.htm, and
http://www.microscopy-uk.org.uk/mag/artdec99/jgpollen.html .